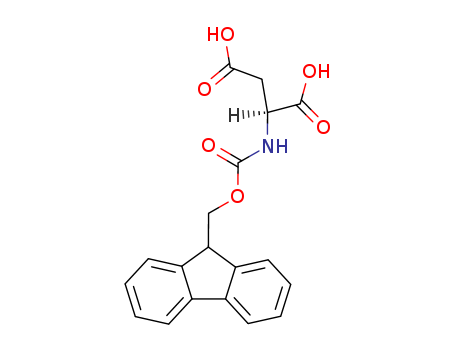
119062-05-4
- Product Name:Fmoc-L-aspartic acid
- Molecular Formula:C19H17NO6
- Purity:99%
- Molecular Weight:355.347
Product Details;
CAS#: 119062-05-4
Molecular Formula: C19H17NO6
Trustworthy Manufacturer Supply High Purity ≥99.5% 119062-05-4 with Cheap Price
- Molecular Formula:C19H17NO6
- Molecular Weight:355.347
- Vapor Pressure:1.24E-14mmHg at 25°C
- Melting Point:180-190 °C
- Refractive Index:1.628
- Boiling Point:587.2 °C at 760 mmHg
- PKA:3.66±0.23(Predicted)
- Flash Point:308.9 °C
- PSA:112.93000
- Density:1.399 g/cm3
- LogP:2.84390
Fmoc-L-aspartic acid(Cas 119062-05-4) Usage
InChI:InChI=1/C19H17NO6/c21-17(22)9-16(18(23)24)20-19(25)26-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15-16H,9-10H2,(H,20,25)(H,21,22)(H,23,24)/t16-/m0/s1
119062-05-4 Relevant articles
Profiling primary protease specificity by peptide synthesis on a solid support
Doeze, Ron H. P.,Maltman, Beatrice A.,Egan, Claire L.,Ulijn, Rein V.,Flitsch, Sabine L.
, p. 3138 - 3141 (2004)
Reverse screening: A greatly simplified ...
Reaction of N-Fmoc aspartic anhydride with glycosylamines: a simple entry to N-glycosyl asparagines
Ibatullin, Farid M.,Selivanov, Stanislav I.
, p. 6351 - 6354 (2009)
The reaction of N-Fmoc-aspartic anhydrid...
A new polymer-supported reagent for the Fmoc-protection of amino acids
Chinchilla, Rafael,Dodsworth, David,Nájera, Carmen,Soriano, José
, p. 7579 - 7581 (2001)
A new polymer-supported Fmoc-OSu (Fmoc-P...
MgI2-Mediated Chemoselective Cleavage of Protecting Groups: An Alternative to Conventional Deprotection Methodologies
Berthet, Mathéo,Davanier, Florian,Dujardin, Gilles,Martinez, Jean,Parrot, Isabelle
supporting information, p. 11014 - 11016 (2015/11/10)
The scope of MgI2 as a valuable tool for...
Ethyl substituted coumarin-4-yl derivatives as photoremovable protecting groups for amino acids with improved stability for SPPS
Weis, Simone,Shafiq, Zahid,Gropeanu, Radu A.,Del Campo, Aránzazu
scheme or table, p. 52 - 57 (2012/09/07)
The synthesis, photochemical properties ...
New TFA-free cleavage and final deprotection in Fmoc solid-phase peptide synthesis: Dilute HCl in fluoro alcohol
Palladino, Pasquale,Stetsenko, Dmitry A.
supporting information, p. 6346 - 6349 (2013/02/25)
A novel method for cleaving from resin a...
A microwave-assisted synthesis of (S)-N-protected homoserine γ-lactones from l-aspartic acid
Singh, Suneel P.,Michaelides, Alex,Merrill, A. Rod,Schwan, Adrian L.
experimental part, p. 6825 - 6831 (2011/10/08)
A three-pot preparation of (S)-N-protect...
119062-05-4 Process route
-
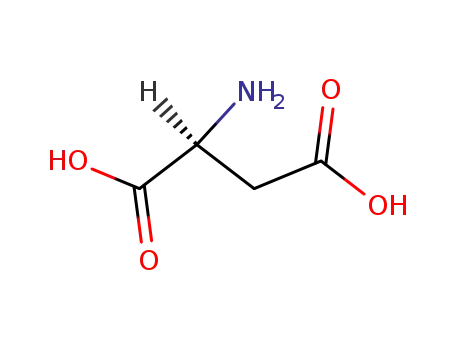
- 56-84-8,25608-40-6,27881-03-4,32505-46-7,52526-39-3
L-Aspartic acid

-
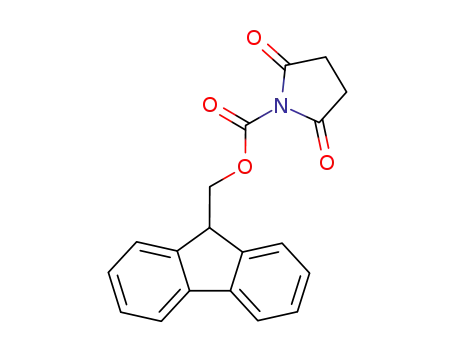
- 102774-86-7
9-fluorenylmethyl N-succinimidyl carbonate

-
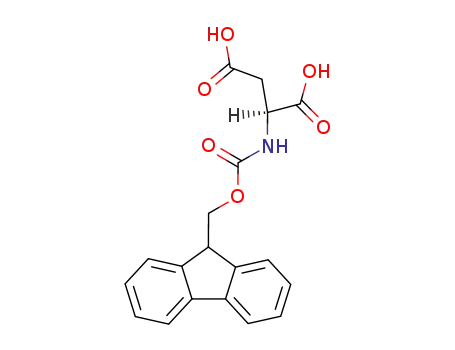
- 136083-57-3,136083-73-3,119062-05-4
N-α-9-fluorenylmethoxycarbonyl-aspartic acid
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In water; N,N-dimethyl-formamide; at 0 - 20 ℃; for 1h;
|
92% |
-
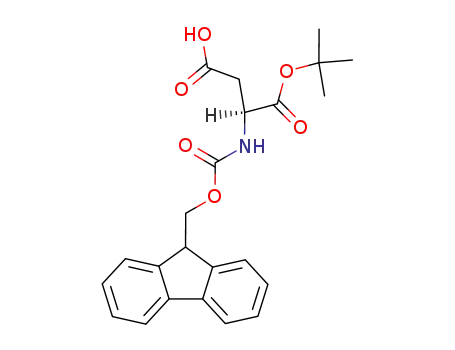
- 129460-09-9,134098-70-7
Fmoc-Asp-O-t-Bu

-

- 136083-57-3,136083-73-3,119062-05-4
N-α-9-fluorenylmethoxycarbonyl-aspartic acid
| Conditions | Yield |
|---|---|
|
With triethylsilane; trifluoroacetic acid; In dichloromethane; for 0.416667h; Ambient temperature;
|
96% |
|
With phosphoric acid; In toluene; at 20 ℃; for 6h;
|
95% |
119062-05-4 Upstream products
-
56-84-8

L-Aspartic acid
-
82911-69-1
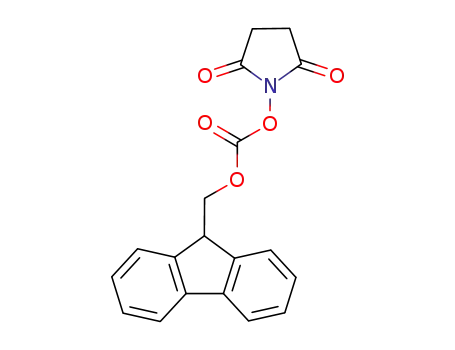
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
-
129460-09-9

Fmoc-Asp-O-t-Bu
-
102774-86-7

9-fluorenylmethyl N-succinimidyl carbonate
Relevant Products
-
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
Boc-L-aspartic acid 4-tert-butyl ester
CAS:1676-90-0
-
Fmoc-L-Proline
CAS:71989-31-6