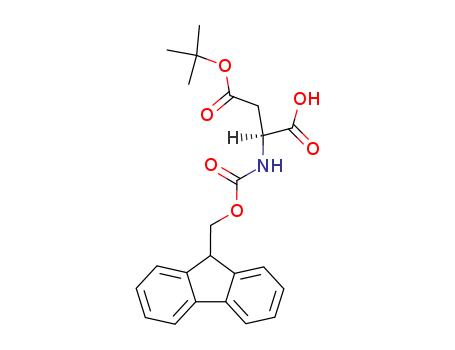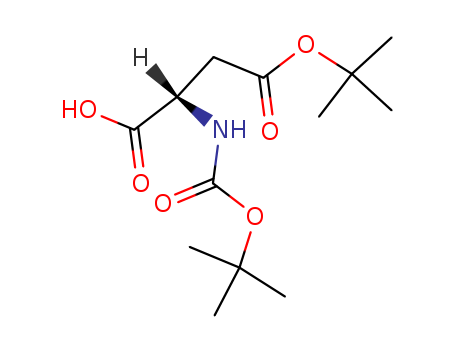
1676-90-0
- Product Name:Boc-L-aspartic acid 4-tert-butyl ester
- Molecular Formula:C13H23NO6
- Purity:99%
- Molecular Weight:289.329
Product Details;
CAS#: 1676-90-0
Molecular Formula: C13H23NO6
Appearance: White to off-white powder
Quality Factory Supply 1676-90-0 with Low Price, Buy High Quality Boc-L-Asp(OtBu)-OH
- Molecular Formula:C13H23NO6
- Molecular Weight:289.329
- Appearance/Colour:White to off-white powder
- Vapor Pressure:1.07E-08mmHg at 25°C
- Melting Point:64-67 °C
- Refractive Index:1.47
- Boiling Point:432.6 °C at 760 mmHg
- PKA:3.69±0.23(Predicted)
- Flash Point:215.4 °C
- PSA:101.93000
- Density:1.139 g/cm3
- LogP:2.08700
Boc-L-aspartic acid 4-tert-butyl ester(Cas 1676-90-0) Usage
InChI:InChI=1/C13H23NO6/c1-12(2,3)19-9(15)7-8(10(16)17)14-11(18)20-13(4,5)6/h8H,7H2,1-6H3,(H,14,18)(H,16,17)/t8-/m0/s1
1676-90-0 Relevant articles
BETA-SUBSTITUTED BETA-AMINO ACIDS AND ANALOGS AS CHEMOTHERAPEUTIC AGENTS AND USES THEREOF
-
Paragraph 0652, (2017/02/28)
β-Substituted β-amino acids, β-substitut...
BETA-SUBSTITUTED BETA-AMINO ACIDS AND ANALOGS AS CHEMOTHERAPEUTIC AGENTS
-
Paragraph 0620, (2015/09/22)
β-Substituted β-amino acids, β-substitut...
γ-Butyrobetaine hydroxylase catalyses a Stevens type rearrangement
Henry, Luc,Leung, Ivanhoe K.H.,Claridge, Timothy D.W.,Schofield, Christopher J.
supporting information; experimental part, p. 4975 - 4978 (2012/09/07)
γ-Butyrobetaine hydroxylase (BBOX) is a ...
METHOD FOR THE SYNTHESIS OF PEPTIDES WITHOUT SOLVENT
-
Page/Page column 5-6, (2010/02/17)
The disclosure relates to a method for t...
1676-90-0 Process route
-
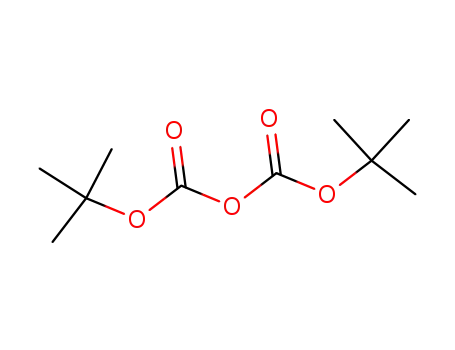
- 24424-99-5
di-tert-butyl dicarbonate

-
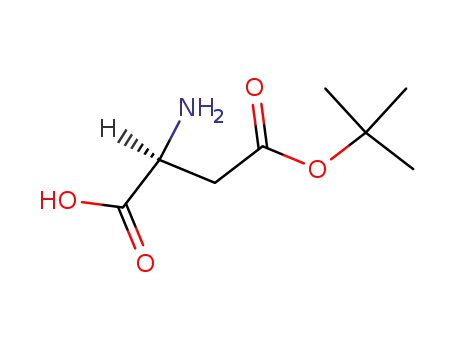
- 3057-74-7
L-Asp(t-Bu)

-
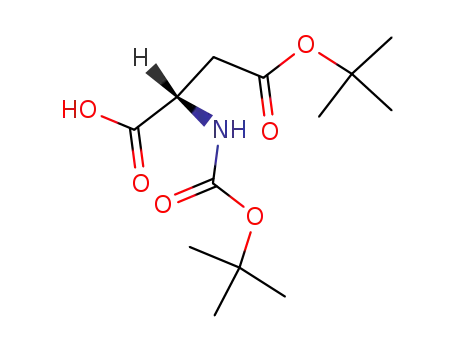
- 1676-90-0
Boc-Asp(OtBu)-OH
| Conditions | Yield |
|---|---|
|
With triethylamine; In 1,4-dioxane; water;
|
100% |
|
L-Asp(t-Bu); In tetrahydrofuran; water; at 0 ℃; for 0.0833333h;
di-tert-butyl dicarbonate; With sodium carbonate; In tetrahydrofuran; water; at 20 ℃; for 24h;
|
97% |
|
With sodium hydroxide; In 1,4-dioxane; water;
|
96% |
|
With barium(II) perchlorate; triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
95% |
-
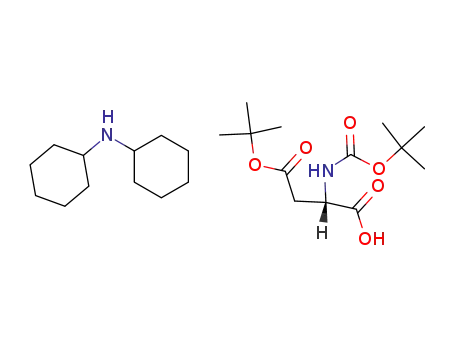
- 1913-12-8,200334-95-8
β-tert-butyl-N-(tert-butoxycarbonyl)-L-aspartic acid dicyclohexylammonium salt

-

- 1676-90-0
Boc-Asp(OtBu)-OH
| Conditions | Yield |
|---|---|
|
With citric acid; In water; ethyl acetate;
|
|
|
β-tert-butyl-N-(tert-butoxycarbonyl)-L-aspartic acid dicyclohexylammonium salt; With sodium hydroxide; water; Ion exchange resin;
With hydrogenchloride; In water; pH=4;
|
1676-90-0 Upstream products
-
1070-19-5
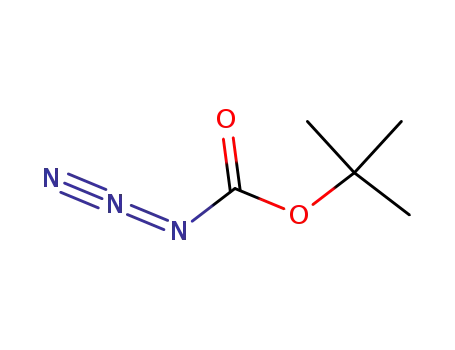
N-(tert-butyloxycarbonyl) azide
-
146692-91-3
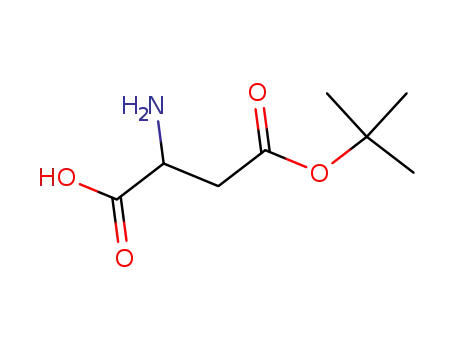
Z-aspartic acid alpha-tert-butyl ester
-
3057-74-7

L-Asp(t-Bu)
-
2673-19-0
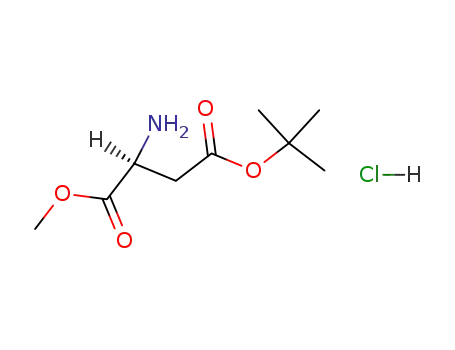
4-tert-butyl 1-methyl L-aspartate hydrochloride
1676-90-0 Downstream products
-
1370749-25-9
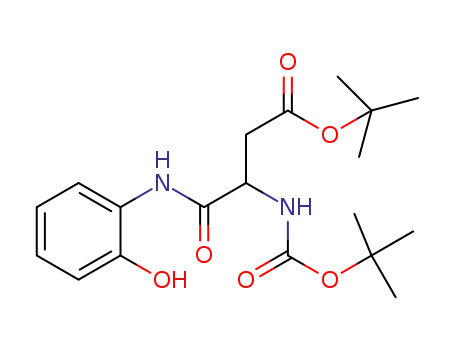
tert-butyl N-[1-(2-hydroxyphenylcarbamoyl)-2-(tert-butoxycarbonyl)ethyl]carbamate
-
1266116-37-3
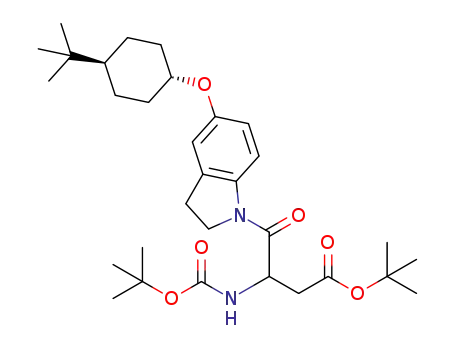
3-tert-butoxycarbonylamino-4-[5-(trans-4-tert-butyl-cyclohexyloxy)-2,3-dihydro-indol-1-yl]-4-oxo-butyric acid tert-butyl ester
-
1266120-58-4
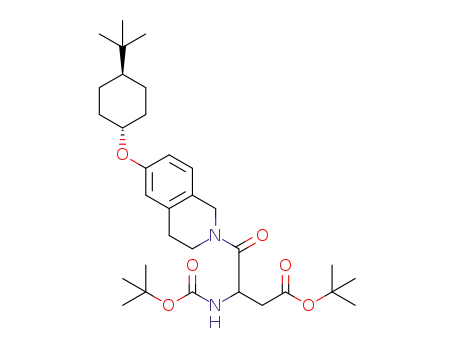
3-tert-butoxycarbonylamino-4-[6-(4-tert-butyl-cyclohexyloxy)-3,4-dihydro-1H-isoquinolin-2-yl]-4-oxo-butyric acid tert-butyl ester
-
115967-22-1
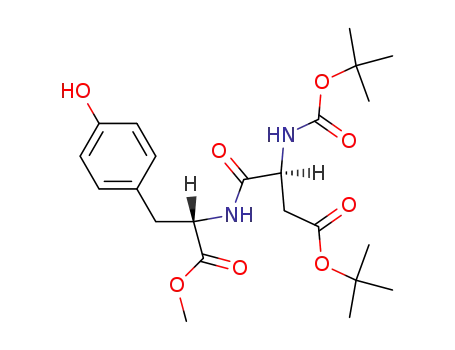
tert-butyloxycarbonyl-β-tert-butyloxyaspartyl-tyrosine α-methyl ester
Relevant Products
-
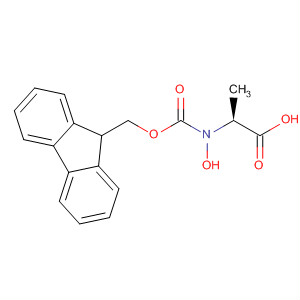
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
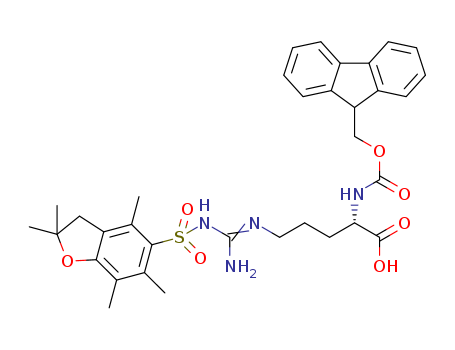
Fmoc-L-citrulline
CAS:133174-15-9
-
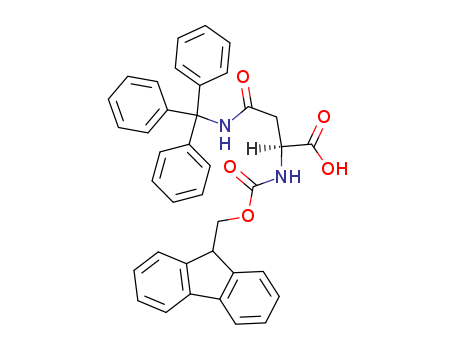
Fmoc-L-aspartic acid
CAS:119062-05-4