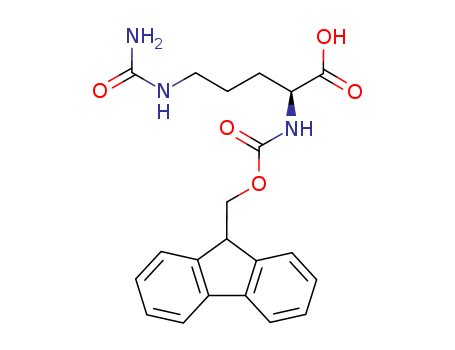
133174-15-9
- Product Name:Fmoc-L-citrulline
- Molecular Formula:C21H23 N3 O5
- Purity:99%
- Molecular Weight:397.431
Product Details;
CAS#: 133174-15-9
Molecular Formula: C21H23 N3 O5
Appearance: White to off white powder
Reputable Manufacturer Supply 133174-15-9 with Cheapest Price, Factory Sells Fmoc-L-Cit-OH
- Molecular Formula:C21H23N3O5
- Molecular Weight:397.431
- Appearance/Colour:White to off white powder
- Vapor Pressure:6.01E-19mmHg at 25°C
- Melting Point:168-175ºC
- Refractive Index:-10 ° (C=1, DMF)
- Boiling Point:671.5oC at 760 mmHg
- PKA:3.84±0.21(Predicted)
- Flash Point:359.9oC
- PSA:130.75000
- Density:1.316g/cm3
- LogP:3.90890
Fmoc-L-citrulline(Cas 133174-15-9) Usage
InChI:InChI=1/C21H23N3O5/c22-20(27)23-11-5-10-18(19(25)26)24-21(28)29-12-17-15-8-3-1-6-13(15)14-7-2-4-9-16(14)17/h1-4,6-9,17-18H,5,10-12H2,(H,24,28)(H,25,26)(H3,22,23,27)/t18-/m0/s1
133174-15-9 Relevant articles
A Wireframe DNA Cube: Antibody Conjugate for Targeted Delivery of Multiple Copies of Monomethyl Auristatin E
M?rcher, Anders,Nijenhuis, Minke A. D.,Gothelf, Kurt V.
supporting information, p. 21691 - 21696 (2021/09/02)
In recent years, several antibody drug c...
Improved Methodology for the Synthesis of a Cathepsin B Cleavable Dipeptide Linker, Widely Used in Antibody-Drug Conjugate Research
Mondal, Deboprosad,Ford, Jacob,Pinney, Kevin G.
supporting information, p. 3594 - 3599 (2018/09/11)
Antibody-drug conjugates (ADCs) represen...
DRUG-LINKER CONJUGATE PHARMACEUTICAL COMPOSITIONS
-
Paragraph 000158, (2017/05/02)
Compositions are disclosed having a cyto...
SILVESTROL ANTIBODY-DRUG CONJUGATES AND METHODS OF USE
-
Page/Page column 81; 82, (2018/01/15)
The invention relates generally to a sil...
133174-15-9 Process route
-
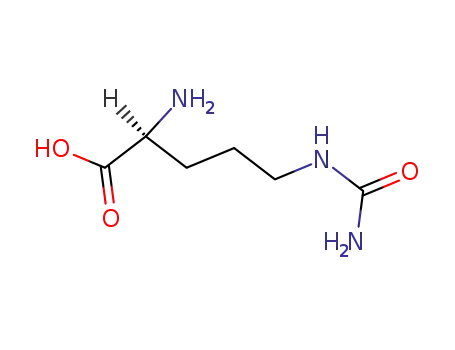
- 372-75-8
Citrulline

-
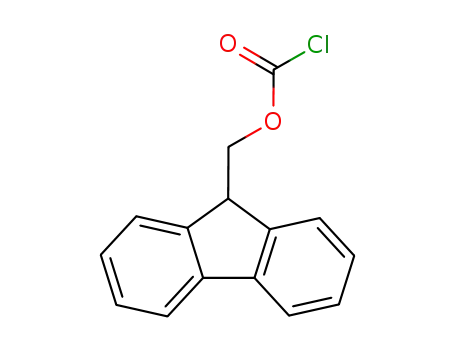
- 28920-43-6
(fluorenylmethoxy)carbonyl chloride

-
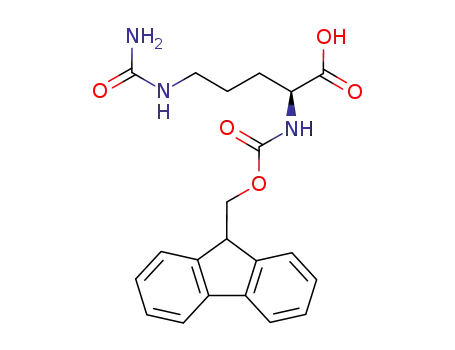
- 133174-15-9
(S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-ureidopentanoic acid
| Conditions | Yield |
|---|---|
|
Citrulline; With sodium hydrogencarbonate; In water; at 20 ℃; for 1h;
(fluorenylmethoxy)carbonyl chloride; In 1,2-dimethoxyethane; water;
|
100% |
|
With sodium hydrogencarbonate; In 1,2-dimethoxyethane; water; at 20 ℃; for 24h; Inert atmosphere;
|
96% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2h;
|
95.6% |
|
With potassium carbonate; In 1,4-dioxane; water; for 2h; Inert atmosphere;
|
95% |
|
With sodium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2.08333h;
|
-
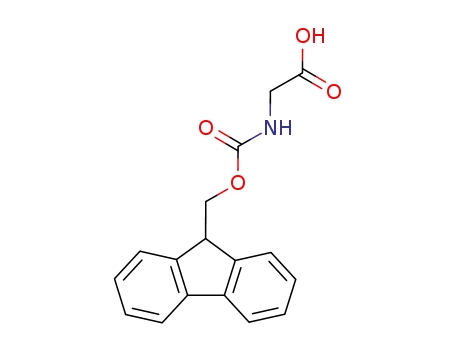
- 29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine

-
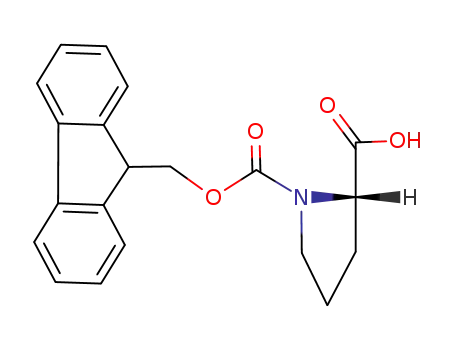
- 71989-31-6
Fmoc-Pro-OH

-
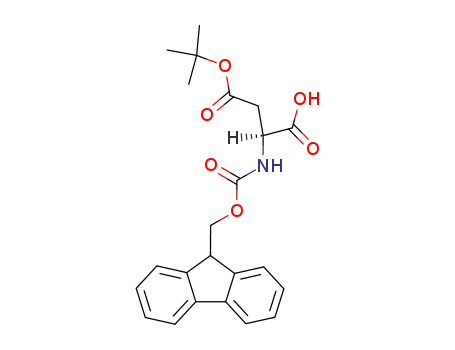
- 71989-14-5
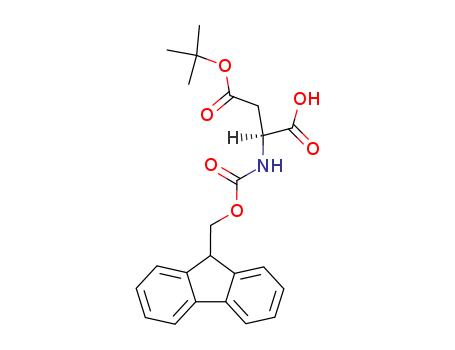
Fmoc-(tBu)Asp-OH

-
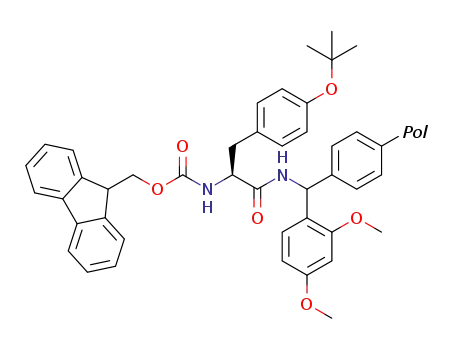
-
C43H43N2O6Pol

-
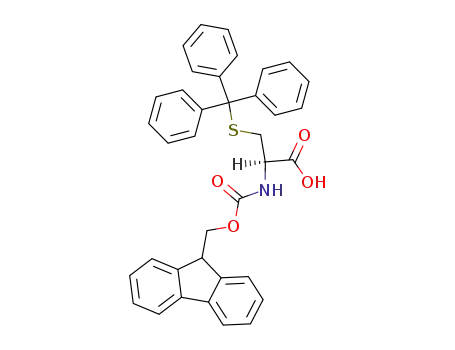
- 103213-32-7,167015-11-4
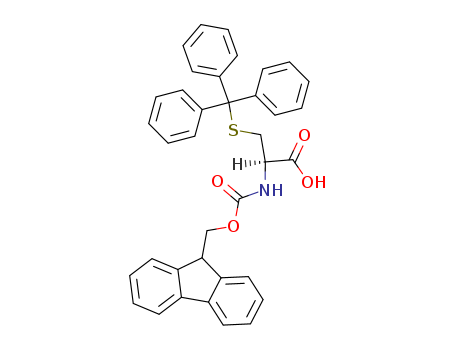
N-(9-fluorenylmethoxycarbonyl)-S-trityl-L-cysteine

-
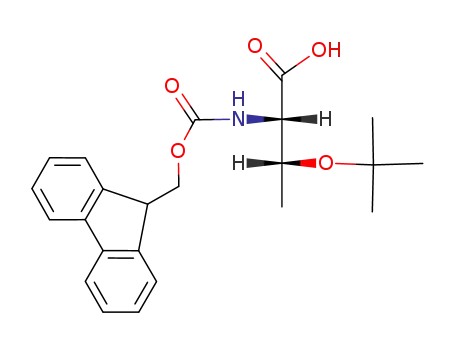
- 71989-35-0,138797-71-4
Fmoc-Thr(tBu)-OH

-
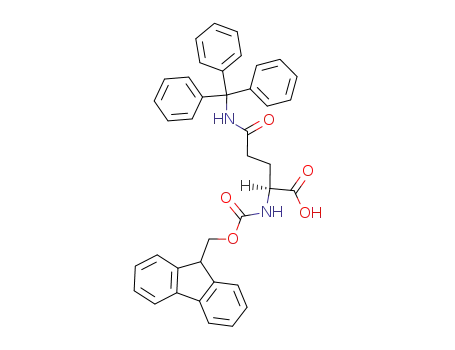
- 132327-80-1,200623-62-7
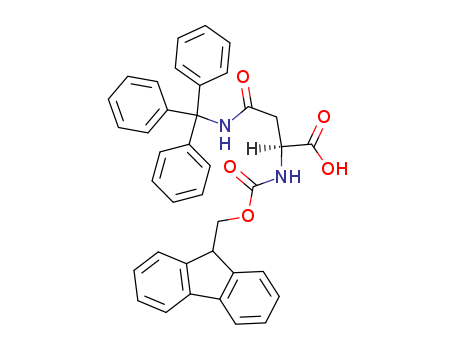
Fmoc-L-Gln(Trt)-OH

-
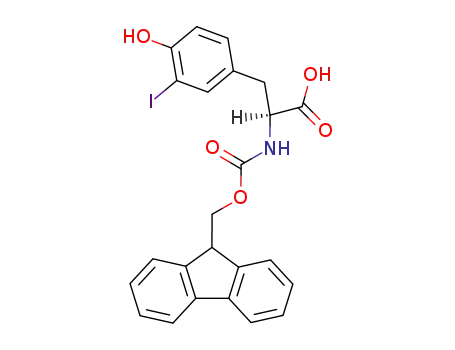
- 134486-00-3,244028-70-4
(S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-(4-hydroxy-3-iodophenyl)propionic acid

-

- 133174-15-9
(S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-ureidopentanoic acid

-
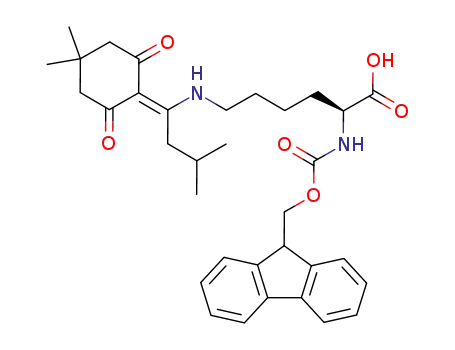
- 204777-78-6
Fmoc-Lys(ivDde)-OH

-
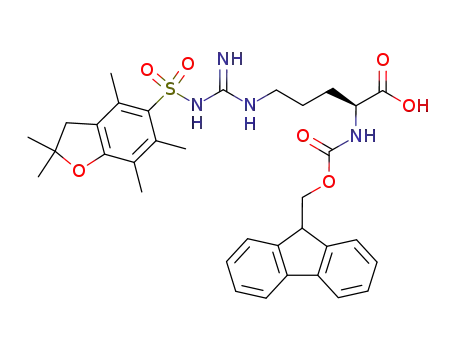
- 187618-60-6
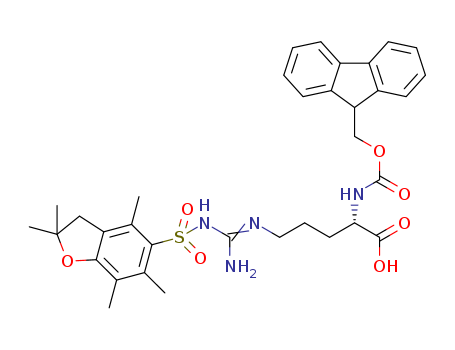
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine

-
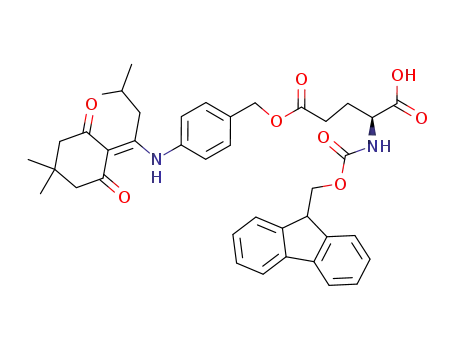
-
Fmoc-Glu(ODmab)-OH

-
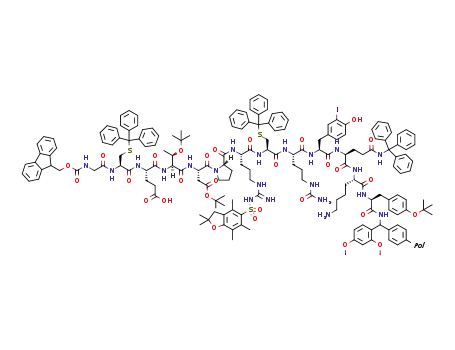
-
C179H207IN21O29PolS3
| Conditions | Yield |
|---|---|
|
C43H43N2O6Pol; With piperidine; In N,N-dimethyl-formamide; for 0.5h; Rink amide MBHA resin
Fmoc-Lys(ivDde)-OH; With 1-hydroxy-7-aza-benzotriazole; N-ethyl-N,N-diisopropylamine; HATU; In N,N-dimethyl-formamide; for 2.5h; Rink amide MBHA resin
N-(fluoren-9-ylmethoxycarbonyl)glycine; Fmoc-Pro-OH; Fmoc-(tBu)Asp-OH; N-(9-fluorenylmethoxycarbonyl)-S-trityl-L-cysteine; Fmoc-Thr(tBu)-OH; Fmoc-L-Gln(Trt)-OH; (S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-(4-hydroxy-3-iodophenyl)propionic acid; (S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-ureidopentanoic acid; Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine; Fmoc-Glu(ODmab)-OH; Further stages;
|
133174-15-9 Upstream products
-
372-75-8

Citrulline
-
28920-43-6

(fluorenylmethoxy)carbonyl chloride
133174-15-9 Downstream products
-
776305-25-0
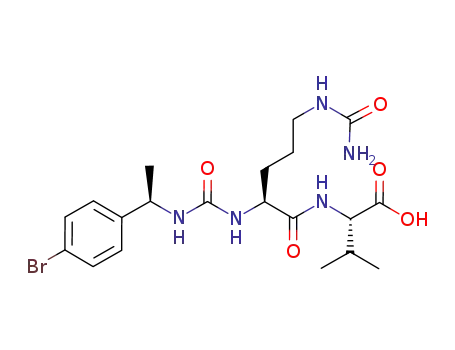
2-(2-{3-[1-(4-bromo-phenyl)-ethyl]-ureido}-5-ureido-pentanoylamino)-3-methyl-butyric acid
-
1007391-44-7
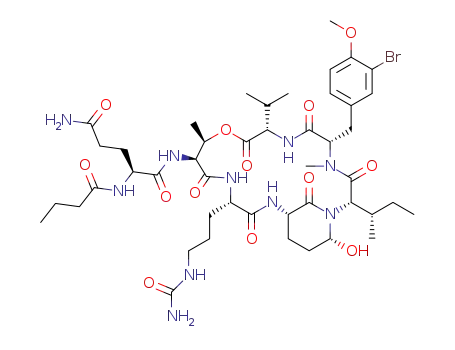
symplocamide A
-
1449785-43-6
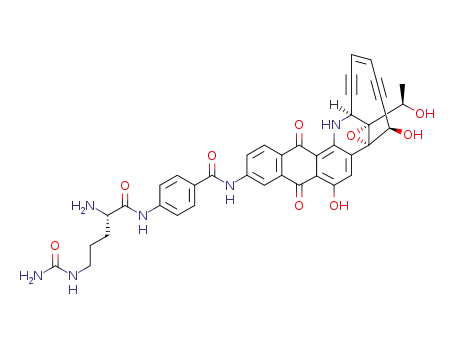
C39H34N6O9
-
1608127-09-8
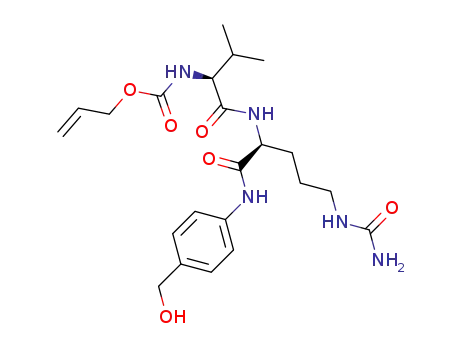
C22H33N5O6
Relevant Products
-
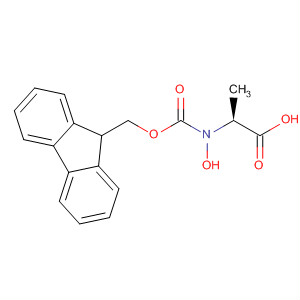
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
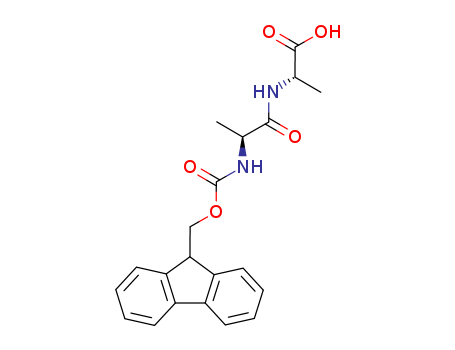
FMOC-ALA-ALA-OH
CAS:87512-31-0
-
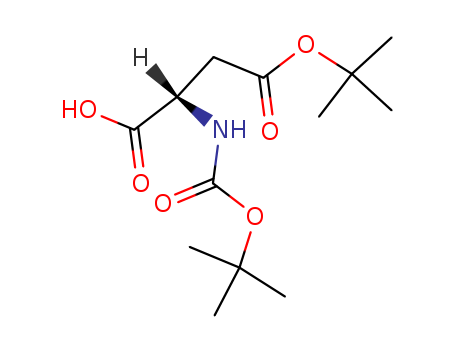
Boc-L-aspartic acid 4-tert-butyl ester
CAS:1676-90-0