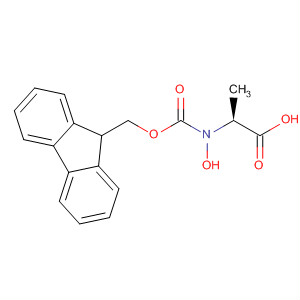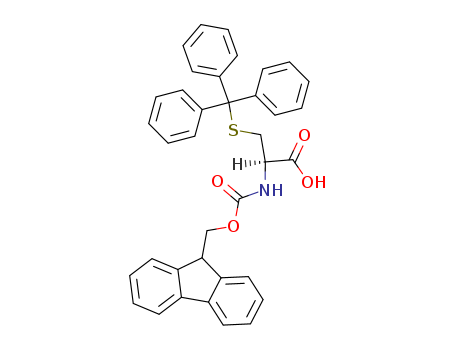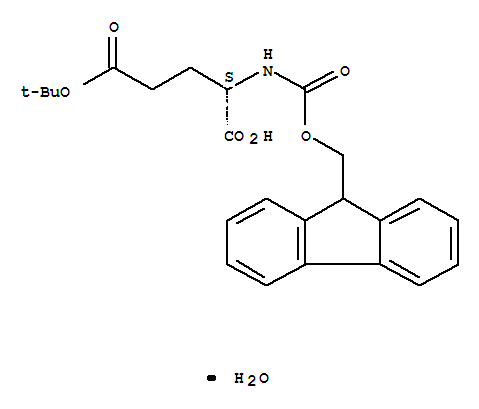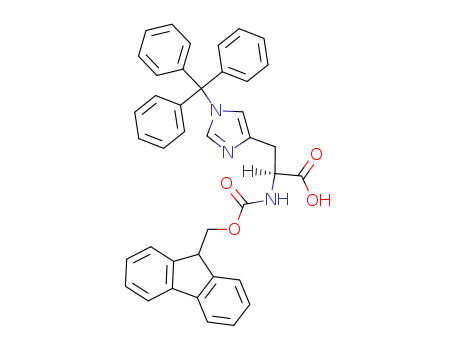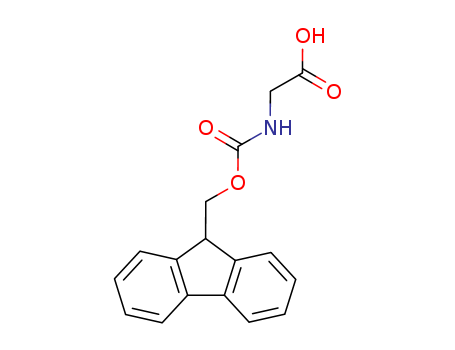
29022-11-5
- Product Name:Fmoc-Glycine
- Molecular Formula:C17H15NO4
- Purity:99%
- Molecular Weight:297.31
Product Details;
CAS#: 29022-11-5
Molecular Formula: C17H15NO4
Appearance: white to light yellow crystal powder
Reputable Factory Supply Hot Sale 29022-11-5 with the Best Price
- Molecular Formula:C17H15NO4
- Molecular Weight:297.31
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:9.69E-13mmHg at 25°C
- Melting Point:174-178 °C
- Refractive Index:1.4500 (estimate)
- Boiling Point:545.7 °C at 760 mmHg
- PKA:3.89±0.10(Predicted)
- Flash Point:283.8 °C
- PSA:75.63000
- Density:1.316 g/cm3
- LogP:3.00060
Fmoc-Glycine(Cas 29022-11-5) Usage
|
General Description |
The product number for this product was previously 04-12-1001.To obtain a certificate of analysis (CoA) of a lot that begins with the letter “A”, please select the option in the right hand menu “Request a COA for Lot#s starting with A”. |
InChI:InChI=1/C17H15NO4/c19-16(20)9-18-17(21)22-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H,18,21)(H,19,20)/p-1
29022-11-5 Relevant articles
Large molecular assembly of amphotericin B formed in ergosterol-containing membrane evidenced by solid-state NMR of intramolecular bridged derivative
Matsumori, Nobuaki,Sawada, Yuri,Murata, Michio
, p. 11977 - 11984 (2006)
Amphotericin B (AmB 1) is known to assem...
Fmoc-Amox, A Suitable Reagent for the Introduction of Fmoc
Kumar, Ashish,Sharma, Anamika,Haimov, Elvira,El-Faham, Ayman,De La Torre, Beatriz G.,Albericio, Fernando
, p. 1533 - 1541 (2017)
Synthesis of most peptides is achieved u...
Synthesis and characterization of a photocleavable collagen-like peptide
Li, Chunqiang,Ornelas, Alfredo,Williams, Kaitlyn N.,Hatch, Kevin A.,Paez, Aurelio,Aguilar, Angela C.,Ellis, Cameron C.,Tasnim, Nishat,Ray, Supriyo,Dirk, Carl W.,Boland, Thomas,Joddar, Binata,Michael, Katja
, p. 1000 - 1013 (2018)
A 34-amino acid long collagen-like pepti...
Oxime carbonates: Novel reagents for the introduction of fmoc and alloc protecting groups, free of side reactions
Khattab, Sherine N.,Subiros-Funosas, Ramon,El-Faham, Ayman,Albericio, Fernando
, p. 3275 - 3280 (2010)
Fmoc and Alloc protecting groups represe...
Fungal Dioxygenase AsqJ Is Promiscuous and Bimodal: Substrate-Directed Formation of Quinolones versus Quinazolinones
Einsiedler, Manuel,Jamieson, Cooper S.,Maskeri, Mark A.,Houk, Kendall N.,Gulder, Tobias A. M.
supporting information, p. 8297 - 8302 (2021/03/01)
Previous studies showed that the FeII/α-...
Dihydroartemisinin and steroid conjugates, and preparation method and application thereof
-
Paragraph 0098-0102, (2019/11/20)
The present invention discloses conjugat...
A 4-OTBS benzyl-based protective group for carboxylic acids
Fang, Zhijun,Li, Yuyao,Xie, Hexin
supporting information, p. 1658 - 1662 (2019/05/29)
Reported herein is a novel 4-OTBS benzyl...
Unwanted hydrolysis or α/β-peptide bond formation: How long should the rate-limiting coupling step take?
Goldschmidt G?z, Viktória,Nagy, Adrienn,Farkas, Viktor,Keszei, Ern?,Perczel, András
, p. 30720 - 30728 (2019/10/28)
Nowadays, in Solid Phase Peptide Synthes...
29022-11-5 Process route
-
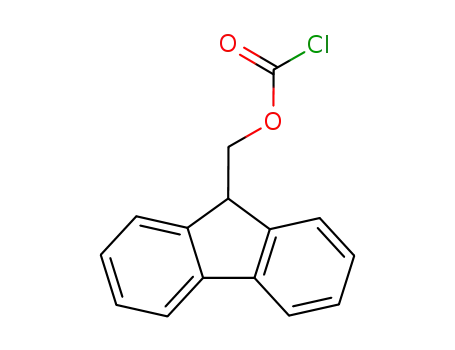
- 28920-43-6
(fluorenylmethoxy)carbonyl chloride

-
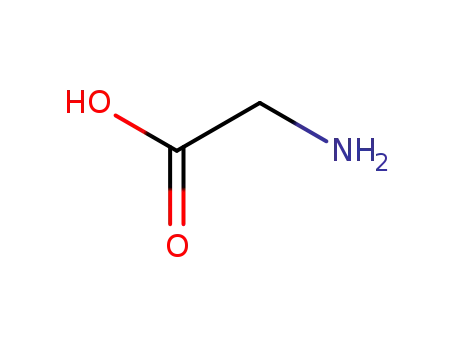
- 56-40-6,18875-39-3,25718-94-9
glycine

-
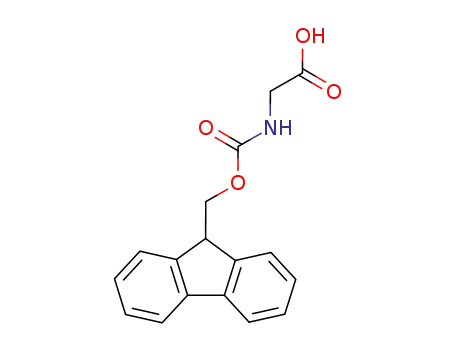
- 29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine
| Conditions | Yield |
|---|---|
|
In ethanol; water; at 60 ℃; for 4h; chemoselective reaction;
|
85% |
|
(fluorenylmethoxy)carbonyl chloride; glycine; With sodium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 4h;
With hydrogenchloride; In water; pH=2;
|
85% |
|
With sodium carbonate; N-ethyl-N,N-diisopropylamine; In water; N,N-dimethyl-formamide; 1.) 0 deg C, 30 min; 2.) up to RT, 1 h;
|
62% |
|
With sodium carbonate;
|
|
|
With sodium carbonate; In 1,4-dioxane; water; for 18h; Ambient temperature;
|
|
|
With N-ethyl-N,N-diisopropylamine;
|
|
|
In acetonitrile; at 20 ℃; for 0.25h; pH=9.2; aq. borate buffer;
|
|
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 2h;
|
|
|
With sodium carbonate; In water; acetone; at 20 ℃; for 2.5h; Cooling with ice;
|
|
|
With potassium carbonate; In 1,4-dioxane; water; at 20 ℃; for 12h;
|
-
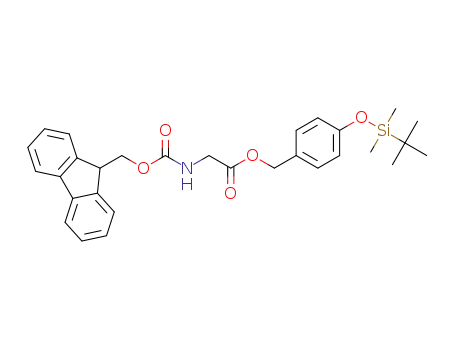
-
4-((tert-butyldimethylsilyl)oxy)benzyl (((9H-fluoren-9-yl)methoxy)carbonyl)glycinate

-

- 29022-11-5
N-(fluoren-9-ylmethoxycarbonyl)glycine
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 0.0833333h; Reagent/catalyst;
|
97% |
29022-11-5 Upstream products
-
28920-43-6

(fluorenylmethoxy)carbonyl chloride
-
56-40-6

glycine
-
100803-86-9
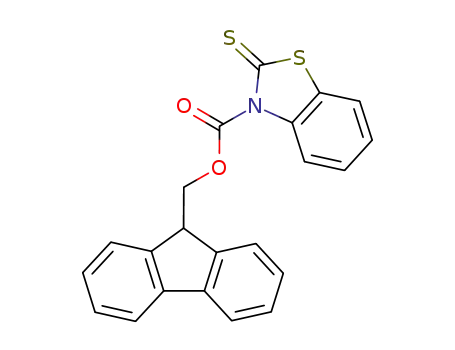
3-(9-Fluorenylmethoxycarbonyl)-benzothiazoline-2-thione
-
88744-04-1
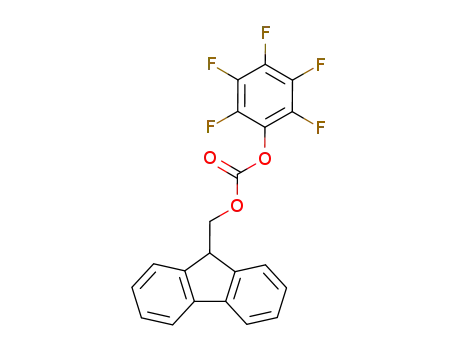
(9-fluorenyl)methyl pentafluorophenyl carbonate
29022-11-5 Downstream products
-
61570-87-4
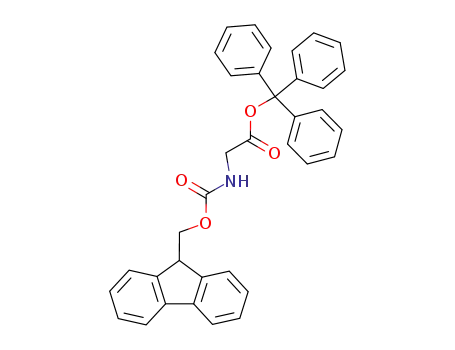
(9H-Fluoren-9-ylmethoxycarbonylamino)-acetic acid trityl ester
-
113484-74-5
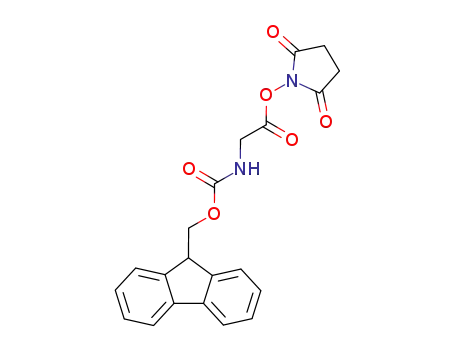
2,5-dioxopyrrolidin-1-yl N-[(9H-fluoren-9-ylmethoxy)carbonyl]glycinate
-
71989-22-5
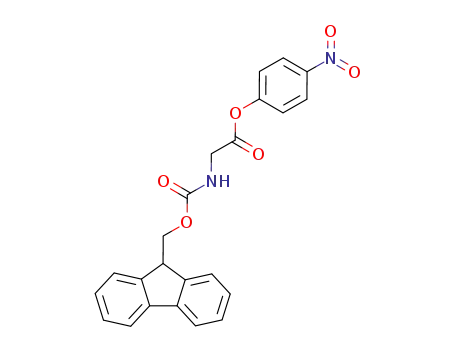
Fmoc-Gly-ONp
-
113534-28-4
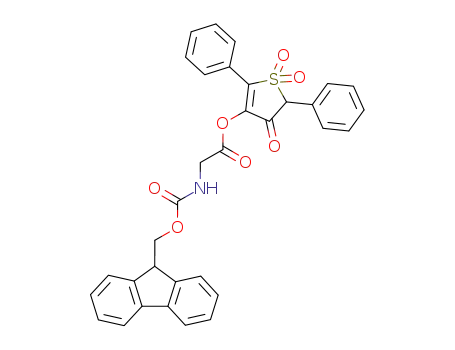
Fmoc-Gly-OTDO
Relevant Products
-
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
FMOC-GLU(OTBU)-OH H2O
CAS:204251-24-1
-
N-Fmoc-N'-trityl-L-histidine
CAS:109425-51-6