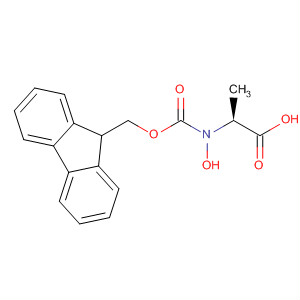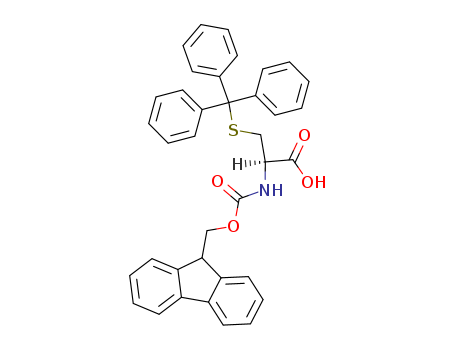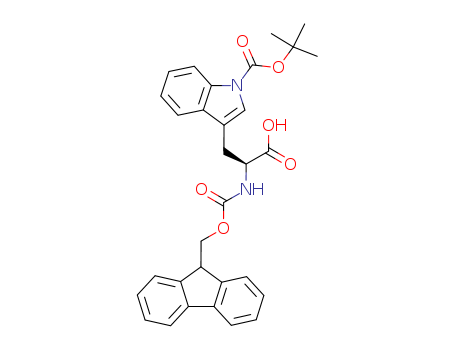
143824-78-6
- Product Name:Fmoc-Trp(Boc)-OH
- Molecular Formula:C31H30N2O6
- Purity:99%
- Molecular Weight:526.589
Product Details;
CAS#: 143824-78-6
Molecular Formula: C31H30N2O6
Appearance: White to off-white crystalline powder
Factory Supply High Purity Hot Sale 143824-78-6 with Competitive Price
- Molecular Formula:C31H30N2O6
- Molecular Weight:526.589
- Appearance/Colour:White to off-white crystalline powder
- Melting Point:86 - 90 °C
- Refractive Index:-20 ° (C=1, DMF)
- PKA:3.71±0.10(Predicted)
- PSA:106.86000
- Density:1.289 g/cm3
- LogP:6.34980
Fmoc-Trp(Boc)-OH(Cas 143824-78-6) Usage
|
General Description |
The use of this N-in-Boc protected derivative overcomes most of the problems associated with the preparation of Trp containing-peptides by Fmoc SPPS [1]. Cleavage with TFA generates an N-in-carboxy indole which protects the Trp from alkylation [1, 2, 3] and sulfonation [1, 4, 5, 6, 7]. The N-in-carboxy group is removed under aqueous conditions during normal work-up of the peptide.The product number for this product was previously 04-12-1103.To obtain a certificate of analysis (CoA) of a lot that begins with the letter “A”, please select the option in the right hand menu “Request a COA for Lot#s starting with A”. |
InChI:InChI=1/C31H30N2O6/c1-31(2,3)39-30(37)33-17-19(20-10-8-9-15-27(20)33)16-26(28(34)35)32-29(36)38-18-25-23-13-6-4-11-21(23)22-12-5-7-14-24(22)25/h4-15,17,25-26H,16,18H2,1-3H3,(H,32,36)(H,34,35)/t26-/m0/s1
143824-78-6 Relevant articles
COMPOUND FOR PREPARATION OF ANTIBODY-PAYLOAD CONJUGATE AND USE THEREOF
-
, (2022/03/15)
The present application relates to a nov...
Method for preparing pharmaceutical intermediate of tryptophan derivative
-
Paragraph 0043; 0045; 0080-0083; 0114-0115, (2021/09/21)
The synthesis method comprises the follo...
DIPHENYLMETHANE COMPOUND
-
, (2010/10/19)
Compounds having a diphenylmethane skele...
Stereoretentive synthesis and chemoselective amide-forming ligations of C-terminal peptide α-ketoacids
Ju, Lei,Lippert, Alexander R.,Bode, Jeffrey W.
, p. 4253 - 4255 (2008/12/20)
C-Terminal peptide cyanosulfur ylides ar...
143824-78-6 Process route
-
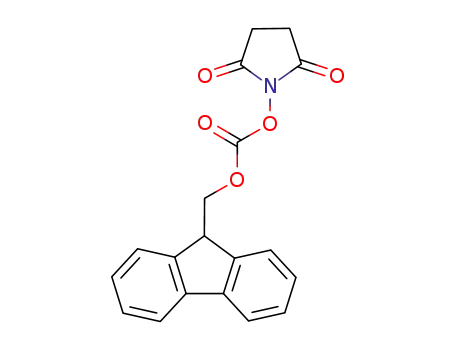
- 82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
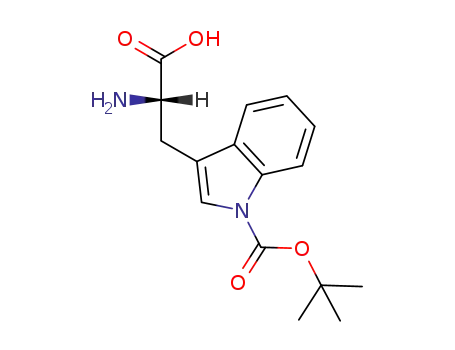
- 146645-63-8
(2S)-2-amino-3-(1-tert-butoxycarbonylindol-3-yl)propanoic acid

-
![3-[(S)-2-carboxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)ethyl]indole-1-carboxylic acid tert-butyl ester](/upload/2024/7/bfb0ee5d-b000-4410-ace5-e14c6cdbe868.png)
- 143824-78-6,163619-04-3
3-[(S)-2-carboxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)ethyl]indole-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20 ℃; Reagent/catalyst; Solvent;
|
80.7% |
-
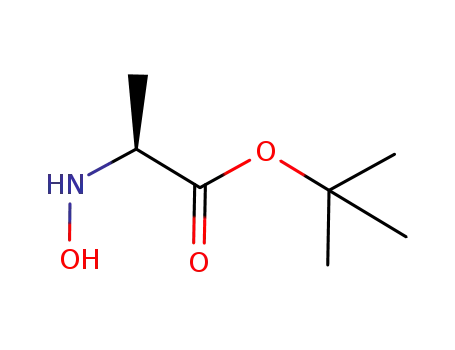
- 83451-16-5,57001-44-2
(S)-tert-butyl 2-(hydroxyamino)propanoate

-
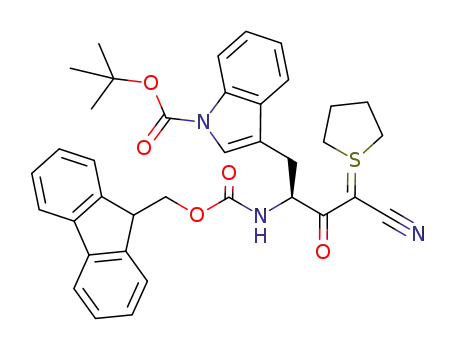
- 1019691-72-5
C37H37N3O5S

-
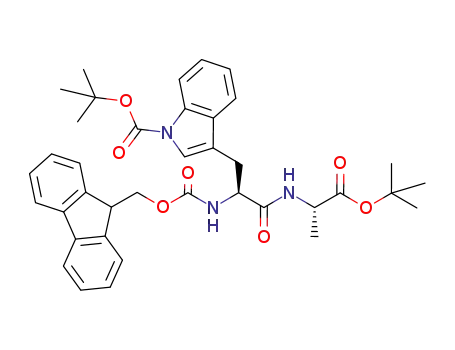
- 1019691-19-0
Fmoc-Trp(Boc)-Ala-OtBu

-
![3-[(S)-2-carboxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)ethyl]indole-1-carboxylic acid tert-butyl ester](/upload/2024/7/bfb0ee5d-b000-4410-ace5-e14c6cdbe868.png)
- 143824-78-6,163619-04-3
3-[(S)-2-carboxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)ethyl]indole-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
C37H37N3O5S; With Oxone; In water; N,N-dimethyl-formamide; at 25 ℃;
(S)-tert-butyl 2-(hydroxyamino)propanoate; In N,N-dimethyl-formamide; at 40 ℃; Further stages.;
|
143824-78-6 Upstream products
-
83451-16-5

(S)-tert-butyl 2-(hydroxyamino)propanoate
-
1019691-72-5

C37H37N3O5S
-
1604-49-5
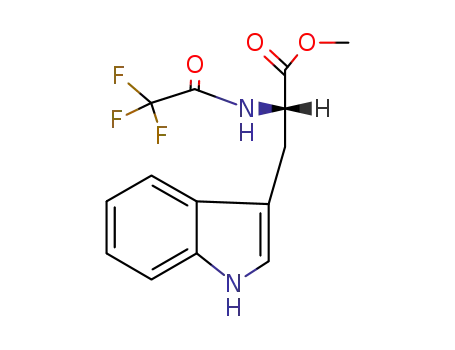
methyl (2S)-3-(1H-indol-3-yl)-2-(trifluoroacetamido)propanoate
-
82911-69-1

N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
Relevant Products
-
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
Fmoc-O-tert-Butyl-L-threonine
CAS:71989-35-0
-
Fmoc-O-tert-butyl-L-tyrosine
CAS:71989-38-3