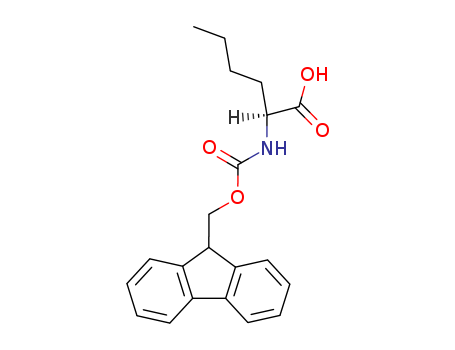
77284-32-3
- Product Name:FMOC-NLE-OH
- Molecular Formula:C21H23NO4
- Purity:99%
- Molecular Weight:353.418
Product Details;
CAS#: 77284-32-3
Molecular Formula: C21H23NO4
Quality Factory Supply Best Quality 77284-32-3 Customized Supply
- Molecular Formula:C21H23NO4
- Molecular Weight:353.418
- Vapor Pressure:1.25E-13mmHg at 25°C
- Melting Point:141-144 °C
- Boiling Point:565.6 °C at 760 mmHg
- PKA:3.91±0.21(Predicted)
- Flash Point:295.9 °C
- PSA:75.63000
- Density:1.209 g/cm3
- LogP:4.55940
FMOC-NLE-OH(Cas 77284-32-3) Usage
InChI:InChI=1/C21H23NO4/c1-2-3-12-19(20(23)24)22-21(25)26-13-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,2-3,12-13H2,1H3,(H,22,25)(H,23,24)/p-1/t19-/m1/s1
77284-32-3 Relevant articles
Determination of Chemical and Enantiomeric Purity of α-Amino Acids and their Methyl Esters as N-Fluorenylmethoxycarbonyl Derivatives Using Amylose-derived Chiral Stationary Phases
Islam, Md. Fokhrul,Adhikari, Suraj,Paik, Man-Jeong,Lee, Wonjae
, p. 332 - 338 (2019/04/13)
Liquid chromatographic enantiomer separa...
AMINO ACID ANALOGUES AND METHODS FOR THEIR SYNTHESIS
-
Page/Page column 48-49, (2014/01/18)
A method for the synthesis of an amino a...
Tandem Ru-alkylidene-catalysed cross metathesis/hydrogenation: Synthesis of lipophilic amino acids
Wang, Zhen J.,Spiccia, Nicolas D.,Jackson, W. Roy,Robinson, Andrea J.
, p. 470 - 476 (2013/08/23)
Highly efficient synthesis of lipidic am...
77284-32-3 Process route
-
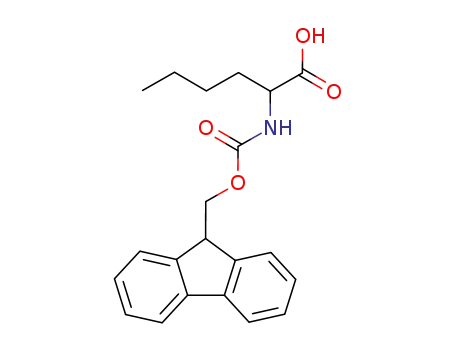
- 144701-20-2
Fmoc-norleucine

-
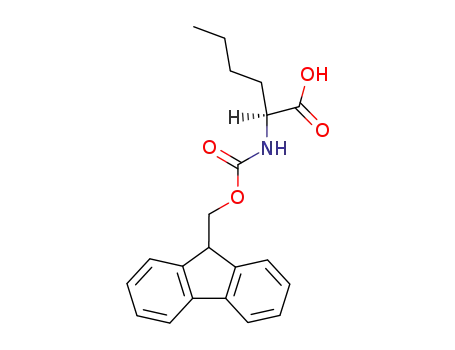
- 77284-32-3,144701-20-2
Fmoc-(S)-2-aminohexanoic acid

-
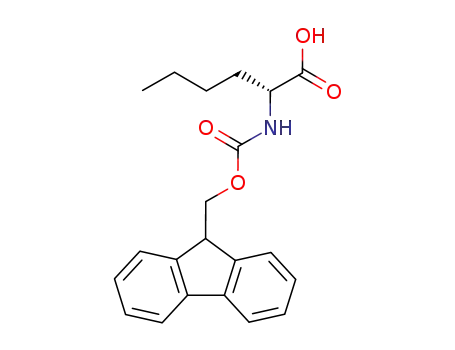
- 112883-41-7,144701-20-2
(R)-((9H-fluoren-9-yl)methoxy)carbonyl-D-norleucine
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; In hexane; isopropyl alcohol; at 25 ℃; Reagent/catalyst; Resolution of racemate;
|
-
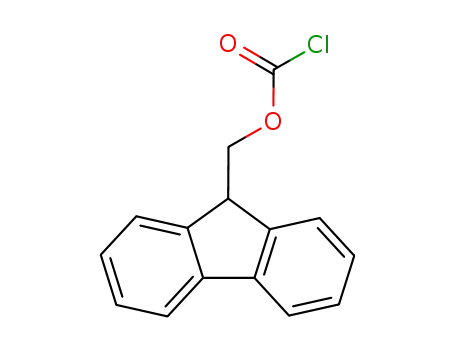
- 28920-43-6
(fluorenylmethoxy)carbonyl chloride

-

- 77284-32-3,144701-20-2
Fmoc-(S)-2-aminohexanoic acid

-

- 112883-41-7,144701-20-2
(R)-((9H-fluoren-9-yl)methoxy)carbonyl-D-norleucine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: sodium carbonate / water; 1,4-dioxane / Cooling with ice
1.2: 5 h / 20 °C
2.1: trifluoroacetic acid / isopropyl alcohol; hexane / 25 °C / Resolution of racemate
With sodium carbonate; trifluoroacetic acid; In 1,4-dioxane; hexane; water; isopropyl alcohol;
|
77284-32-3 Upstream products
-
947154-63-4
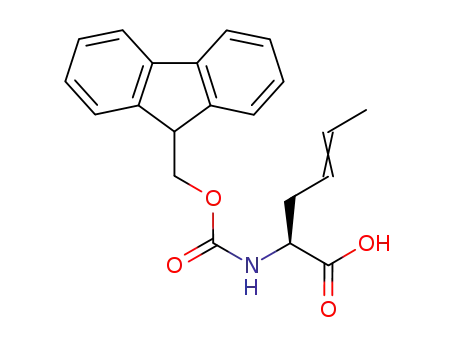
2-N-fluorenylmethoxycarbonylaminohex-4-enoic acid
-
28920-43-6

(fluorenylmethoxy)carbonyl chloride
-
327-57-1
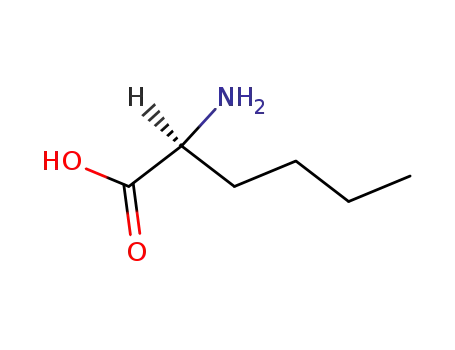
L-Norleucine
-
144701-20-2

Fmoc-norleucine
77284-32-3 Downstream products
-
113534-33-1
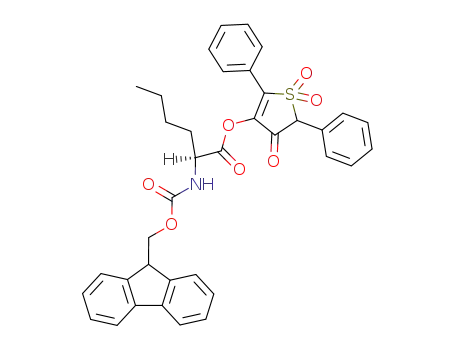
Fmoc-Nle-OTDO
Relevant Products
-
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
OCTADECANEDIOIC ACID MONO-TERT-BUTYL ESTER
CAS:843666-40-0
-
Fmoc-L-Serine
CAS:73724-45-5