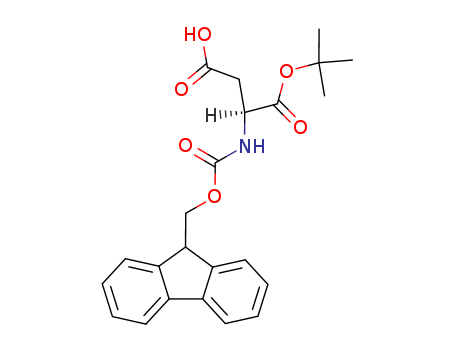
129460-09-9
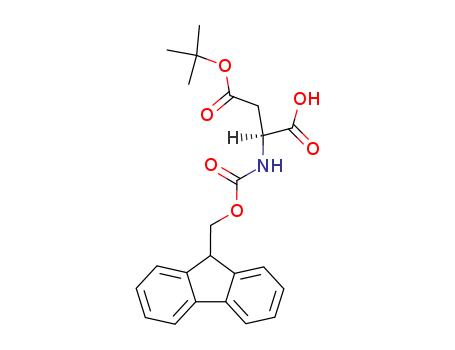
- Product Name:L-Fmoc-Aspartic acid alpha-tert-butyl ester
- Molecular Formula:C23H25NO6
- Purity:99%
- Molecular Weight:411.455
Product Details;
CAS#: 129460-09-9
Molecular Formula: C23H25NO6
Trustworthy Factory Supply 129460-09-9 with Low Price, Buy High Quality Fmoc-L-Asp-OtBu
- Molecular Formula:C23H25NO6
- Molecular Weight:411.455
- Vapor Pressure:4.21E-16mmHg at 25°C
- Melting Point:90-98°C
- Refractive Index:1.576
- Boiling Point:617.4 °C at 760 mmHg
- PKA:4.08±0.19(Predicted)
- Flash Point:327.2 °C
- PSA:101.93000
- Density:1.251 g/cm3
- LogP:4.10100
L-Fmoc-Aspartic acid alpha-tert-butyl ester(Cas 129460-09-9) Usage
InChI:InChI=1/C23H25NO6/c1-23(2,3)30-21(27)19(12-20(25)26)24-22(28)29-13-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,24,28)(H,25,26)/t19-/m0/s1
129460-09-9 Relevant articles
Application of tert-Butyl Disulfide-Protected Amino Acids for the Fmoc Solid-Phase Synthesis of Lactam Cyclic Peptides under Mild Metal-Free Conditions
Chen, Junyou,Cui, Tingting,Sun, Shuaishuai,Guo, Yanyan,Chen, Jingnan,Wang, Jun,Bierer, Donald,Li, Yi-Ming
, p. 8610 - 8619 (2021)
Lactam cyclic peptides are a class of in...
Preparation method of aspartic acid-1-tert-butyl ester derivative
-
Paragraph 0063, (2017/05/02)
The invention relates to a preparation m...
Highly reactive and chemoselective cleavage of allyl esters using an air- and moisture-stable [CpRu(IV)(π-C3H5)(2-quinolinecarboxylato)]PF6 catalyst
Tanaka, Shinji,Saburi, Hajime,Murase, Takanori,Ishibashi, Yoshitaka,Kitamura, Masato
, p. 295 - 298 (2008/02/03)
A new catalytic process for allyl ester ...
(P(C6H5)3)CpRu+-catalyzed deprotection of allyl carboxylic esters
Kitamura, Masato,Tanaka, Shinji,Yoshimura, Masahiro
, p. 4975 - 4977 (2007/10/03)
A new and efficient catalytic method for...
129460-09-9 Process route
-
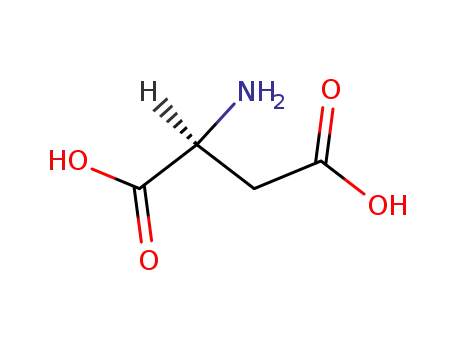
- 56-84-8,25608-40-6,27881-03-4,32505-46-7,52526-39-3
L-Aspartic acid

-
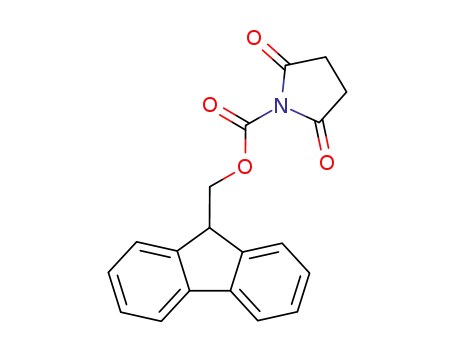
- 102774-86-7
9-fluorenylmethyl N-succinimidyl carbonate

-
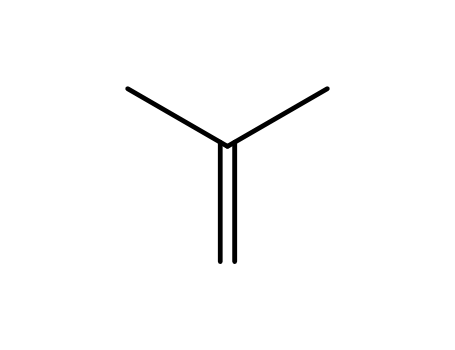
- 115-11-7,15220-85-6
isobutene

-
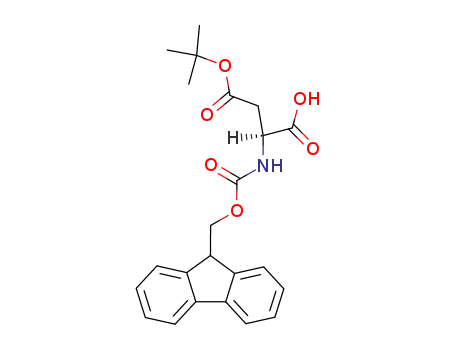
- 71989-14-5
Fmoc-(tBu)Asp-OH

-
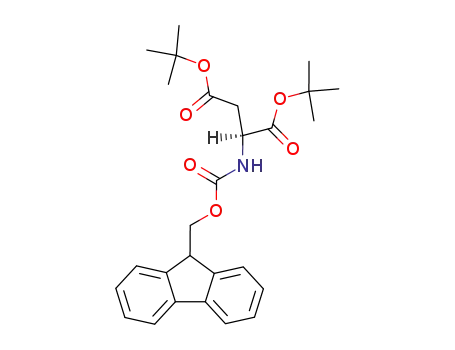
- 129460-17-9
N-(9-fluorenylmethoxycarbonyl)aspartic acid di-tert-butyl ester

-
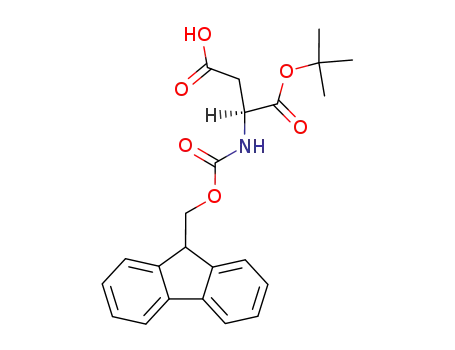
- 129460-09-9,134098-70-7
Fmoc-Asp-O-t-Bu
| Conditions | Yield |
|---|---|
|
With sodium carbonate; toluene-4-sulfonic acid; Yield given. Multistep reaction. Yields of byproduct given; 1) dioxane, gas pressure: 350 mbar, 3 d, r.t., 2) dioxane, 0 deg C to r.t., 14 h;
|
-
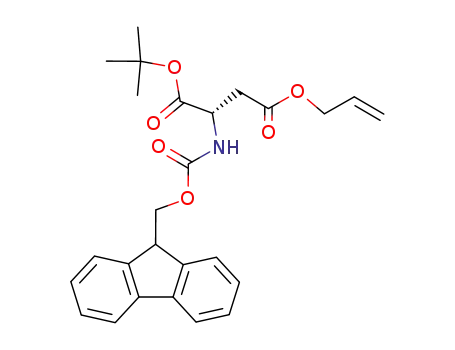
-
2-(9H-fluoren-9-ylmethoxycarbonylamino)-succinic acid 4-allyl ester 1-tert-butyl ester

-

- 129460-09-9,134098-70-7
Fmoc-Asp-O-t-Bu
| Conditions | Yield |
|---|---|
|
[(cyclopentadienyl)bis(acetonitrile)(triphenylphosphine)ruthenium(II)] hexafluorophosphate; In methanol; at 25 ℃; for 17h;
|
99% |
|
[RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid; In methanol; at 30 ℃; for 1h;
|
99 % Spectr. |
129460-09-9 Upstream products
-
56-84-8

L-Aspartic acid
-
102774-86-7

9-fluorenylmethyl N-succinimidyl carbonate
-
115-11-7

isobutene
-
168261-64-1
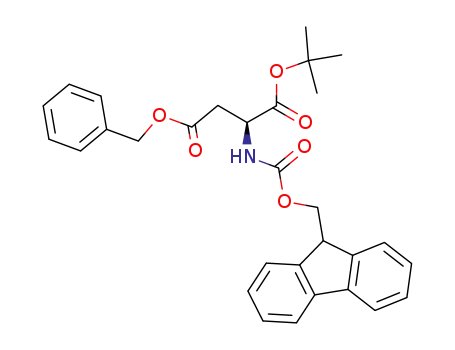
(S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)succinic acid 4-benzyl ester 1-tert-butyl ester
129460-09-9 Downstream products
-
158519-34-7
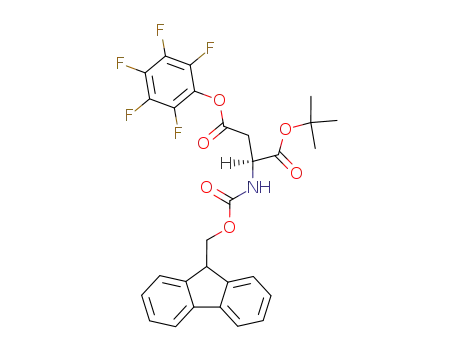
Nα-fluorenylmethoxycarbonyl-L-aspartic acid α-tert-butyl-β-pentafluorophenyl ester
-
136083-57-3
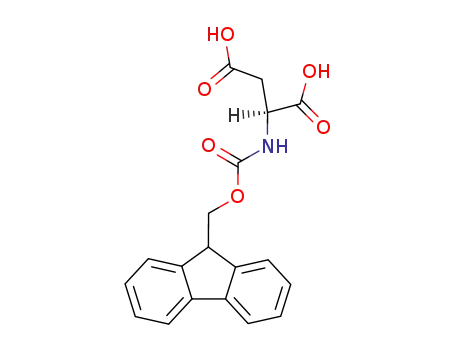
N-α-9-fluorenylmethoxycarbonyl-aspartic acid
-
223480-00-0
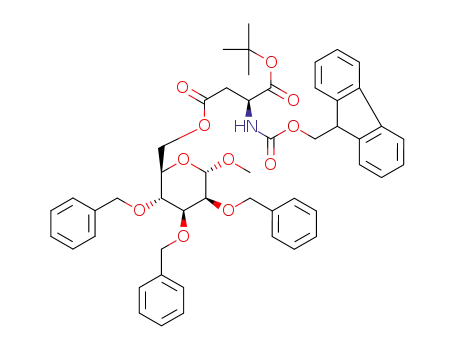
methyl-6-O-carboxy-(N-(9-fluorenylmethoxycarbonyl)-L-aspartic-acid α-tert-butyl ester)-2,3,4-tri-O-benzyl-α-D-manno-pyranoside
-
223480-02-2
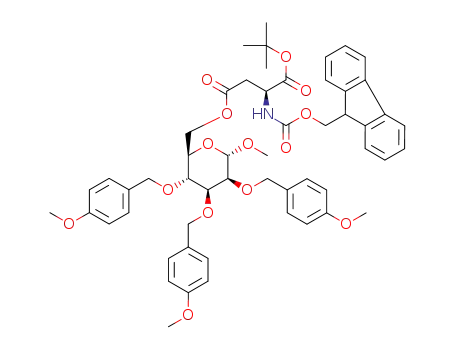
methyl-6-O-carboxy-(N-(9-fluorenylmethoxycarbonyl)-L-aspartic-acid α-tert-butyl ester)-2,3,4-tri-O-4-methoxybenzyl-α-D-manno-pyranoside
Relevant Products
-
Fmoc-L-Ala-OH·H2O
CAS:207291-76-7
-
Fmoc-S-acetamidomethyl-L-cysteine
CAS:86060-81-3